Effective Ways to Name Ionic Compounds
Understanding Ionic Bonds and Cation Naming
Naming ionic compounds involves a systematic approach to understanding the constituents that form ionic bonds. An ionic bond forms between cations (positively charged ions) and anions (negatively charged ions). The first step in naming these compounds is to identify the cation, which can be either a monatomic ion or a polyatomic ion. For monatomic cations, the name is simply the name of the element followed by the word “ion.” For example, sodium becomes sodium ion. In contrast, when dealing with transition metals, the cation naming requires the use of **Roman numerals** to indicate the charge, making it critical to understand the **valency in ionic compounds**.
Differences in Cation and Anion Names
The differences between cation and anion naming are fundamental in ionic nomenclature. While cations retain their elemental name with the addition of “ion”, anions are typically named by modifying the element’s name to reflect its ionic state, usually by adding an “-ide” suffix for monatomic anions. For instance, chlorine becomes chloride when it forms an anion. Polyatomic ions, on the other hand, have unique names that do not necessarily follow this pattern. It is vital for students of chemistry to memorize the names and formulas of common **polyatomic ions** as they appear frequently in naming ionic compounds.
Using Roman Numerals in Naming Transition Metals
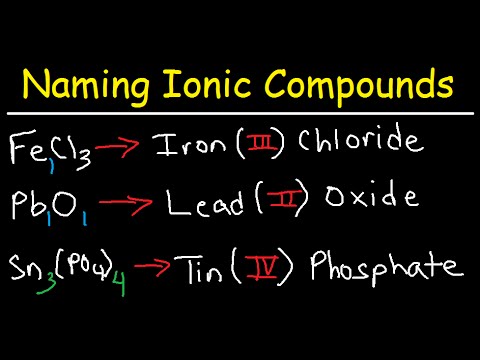
Transition metals are known for their ability to form more than one positive ion. Thus, the use of Roman numerals becomes essential for accurate **naming ionic compounds** involving these elements. For example, iron can form Fe²⁺ and Fe³⁺; therefore, one would refer to them as iron(II) ion and iron(III) ion, respectively, when naming associated compounds. This approach simplifies recognizing the exact charge of the metal in the compound and prevents confusion, ultimately enhancing communication in chemical literature. Understanding **transition metals naming** is an essential skill for anyone studying chemistry, as it appears in many **ionic compound examples**.
Naming Anions and Polyatomic Ions
When naming ionic compounds, it is equally important to understand how to name anions. These negatively charged ions can be either monatomic or polyatomic. Monatomic anions take their name from the element with “-ide” at the end as previously discussed, while naming for polyatomic ions must refer to specific nomenclature rules. Recognizing commonly used polyatomic ions can prevent errors during compilation of chemical formulas and enhances the accuracy of ionic compound identification.
Naming Compounds with Polyatomic Ions
Polyatomic ions have specific names that differ from simple alterations of their elemental names. For example, the sulfate ion is SO₄²⁻, which retains its unique identity compared to sulfate’s more straightforward relatives like chlorate ClO₃⁻. When combining polyatomic ions with monatomic cations, the overall compound name reflects both components. For instance, combining sodium (Na⁺) with sulfate (SO₄²⁻) yields sodium sulfate. Understanding the **naming rules for polyatomic ions** is critical in accurately writing chemical formulas and recognizing compound names.
Common Mistakes in Naming Ionic Compounds
Despite the structured rules governing ionic nomenclature, common mistakes still arise. One frequent error involves incorrect charges during the composition of ionic compounds. A proper grasp of the **charges of ions** is paramount; forgetting or misapplying ion charges can lead to erroneous naming conventions. Repeated practice with **ionic compound formation** and examples can help reinforce correct usage, and familiarize students with creating compounds based on their ionic properties.
Nomenclature Rules for Acids and Bases
In addition to the rules for naming ionic compounds, familiarizing oneself with how to name acids and bases is equally valuable. Acids can be derived from ionic compounds containing anions and **naming rules for acids** depend on whether the acid contains oxygen. Non-oxygen acids typically adopt the “hydro-” prefix. For example, hydrochloric acid (HCl) would reflect its ionic nature derived from the chloride ion. For acids with oxyanions, the nomenclature reflects the polyatomic ion’s name—sulfuric acid stems from sulfate.
Naming Basics for Bases
Understanding the relationship between bases and ionic compounds also sheds light on the broader topic of **naming bases**. Bases usually comprise a metal cation and hydroxide (OH⁻). Their names follow a structure similar to ionic compounds; for instance, sodium hydroxide directly indicates sodium as the cation and hydroxide as the anion. Recognizing the distinction between acids, bases, and salts is essential for approaching broader topics such as **solubility rules** in ionic compounds.
Importance of Correct Naming
Correct naming conventions impact scientific communication and education significantly. Utilizing proper **chemical nomenclature** ensures that each compound can be universally recognized and understood within the scientific community. This clarity is paramount, especially in areas such as pharmaceutical research where precise ionic identities are crucial for product formulation. Therefore, learners and professionals in chemistry are encouraged to notice these naming conventions and apply them diligently in practice.
Key Takeaways
- Understanding both cation and anion naming is essential for accurate naming of ionic compounds.
- Using Roman numerals is crucial for the correct naming of transition metals.
- Recognizing polyatomic ions greatly aids in the proper naming of complex ionic compounds.
- The naming rules for acids and bases complement the overall understanding of ionic nomenclature.
- Ensuring accuracy in naming contributes to effective scientific communication.
FAQ
1. What is the role of charges of ions in naming ionic compounds?
The **charges of ions** direct the naming of ionic compounds by determining how cations and anions combine. Accurately reflecting these charges gives rise to the correct compound name and ensures a precise chemical representation.
2. How do you name hydrated ionic compounds?
Hydrated ionic compounds are named by combining the salt name with a prefix that indicates the number of water molecules. For example, CuSO₄·5H₂O is called copper(II) sulfate pentahydrate. The “penta-” refers to the five water molecules involved.
3. Why is nomenclature important in chemistry?
Nomenclature is vital as it provides a standardized method for identifying compounds, facilitating effective communication among chemists across the globe and avoiding confusion caused by common naming discrepancies.
4. What are some common misconceptions about naming ionic compounds?
One misconception is that all ionic compounds can be named simply by adding “-ide” to the anion. This is untrue, especially when dealing with polyatomic ions or transition metals where more nuanced naming rules apply.
5. How can students improve their skills in naming ionic compounds?
Students can enhance their naming skills by practicing with worksheets, flashcards, and engaging in **classroom activities for teaching naming** conventions. Real-world examples from chemistry can also serve as valuable referencing materials.
By continually revisiting these principles and applying them both in academic and real-world contexts, individuals will become adept at the science of naming ionic compounds.