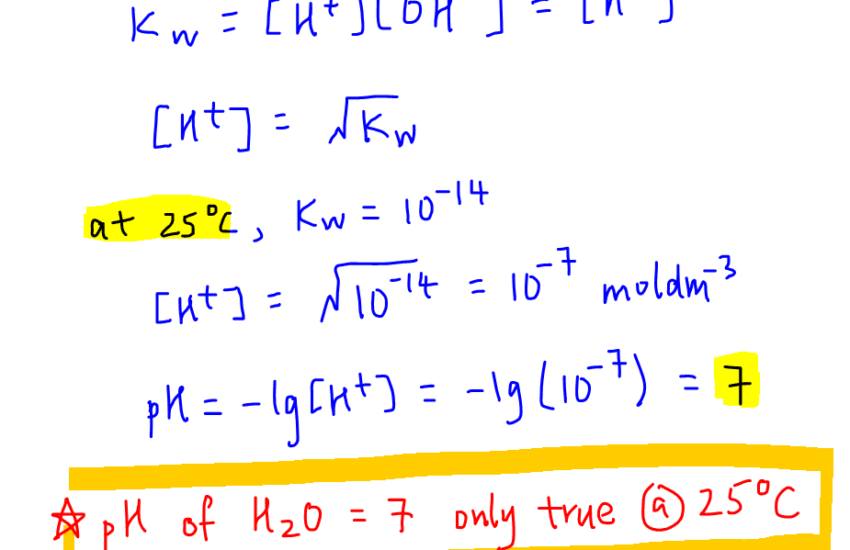“`html
How to Calculate pH: A Simple Guide with Practical Methods for Accurate Results in 2025

Understanding the pH Scale
The pH scale is a numerical scale used to specify the acidity or basicity of a solution. It ranges from 0 to 14, with 7 being considered neutral. A pH less than 7 indicates acidity, while a pH greater than 7 signifies alkalinity. Understanding how to calculate pH is essential for various fields such as biology, chemistry, and environmental science. Detailed knowledge of the importance of pH allows us to accurately assess various solutions, from soil samples to swimming pools. For example, the pH levels in aquariums must be carefully monitored to maintain a healthy environment for aquatic life.
What is the pH Formula?
The pH formula is derived from the equation pH = -log[H+], where [H+] is the concentration of hydrogen ions in moles per liter. This logarithmic scale provides a way to express the acidity or alkalinity of a solution quantitatively. For instance, a solution with [H+] of 0.001 moles per liter has a pH of 3, indicating it is acidic. Conversely, a substance with a lower concentration of hydrogen ions, like baking soda, will demonstrate a basic pH level, typically greater than 7. The logarithmic nature of the pH scale means that a change of one pH unit corresponds to a tenfold change in hydrogen ion concentration.
Importance of the pH in Different Contexts
The significance of pH extends to several domains, influencing chemical reactions, biological processes, and environmental conditions. In agriculture, for example, soil pH directly affects nutrient availability to plants, impacting their growth and productivity. Understanding the effects of pH can significantly enhance crop yields by optimizing what is known as buffer solutions to maintain a stable pH level in the soil. Similarly, in swimming pools, proper pH regulation not only ensures safety but also helps prevent skin and eye irritation.
Methods for Measuring pH
There are several methods for measuring pH, and choosing the right one can lead to more accurate results. Depending on the required precision and context, you may employ pH meters, pH strips, or pH indicators for your measurements.
Using a pH Meter
The pH meter is one of the most precise tools for pH testing. This electronic device uses a glass electrode to measure the voltage difference generated by the hydrogen ions present in the solution, providing a digital pH reading. For optimal accuracy, it is crucial to calibrate the pH meter using standard buffer solutions before use. Additionally, temperature can influence pH readings, so taking that into account during your measurements is vital for accuracy.
pH Strips and Indicators
pH strips and chemical pH indicators provide a more direct yet less precise method of determining pH levels. These strips change color based on the acidity or alkalinity of the solution they are dipped into. While they are less sensitive than pH meters, they are valuable for quick assessments or field work where immediate results are required. It is essential to read the color chart provided with the strips for accurate interpretation.
Practical Example: pH Testing in Soil
When testing pH in soil, it’s crucial to utilize the right method for accurate data. Using a reputable soil test kit will usually involve mixing soil with distilled water and using a pH meter or strip to gauge the result. A common practice is to mix a 1:1 ratio of soil to water, and after letting it settle, insert the meter’s electrode or dip the strip. This gives a quick perspective on how acidic or alkaline your soil is, which can guide your amendments for optimal plant growth based on the importance of pH balance in biology.
Applications of pH in Everyday Life
pH measurement goes beyond the laboratory and has widespread applications in daily life and various industries. From maintaining healthy aquariums to ensuring the edibility and safety of foods, understanding pH plays a crucial role in our lives.
pH in Food and Cooking
The pH in food production impacts flavor, preservation, and even nutrient extraction. For example, various fermentation processes, like brewing beer or making yogurt, depend heavily on the right pH levels to enhance flavor and texture. Moreover, monitoring the acidity and alkalinity when cooking can affect not only taste but also the cooking process itself. Certain ingredients are added not just for flavor but also to correct pH, influencing how well something like bread rises.
Monitoring pH in Aquariums
Aquarium enthusiasts must be aware of the precise pH for aquariums due to its impact on aquatic life. Tropical fish often thrive at pH levels around 6.5 to 7.5. Regular pH testing helps ensure that aquatic conditions are stable. When deviations occur, often remedied through pH adjustments using commercial products or naturally with certain types of driftwood and rocks that can stabilize pH levels.
pH in Swimming Pools
Another practical application is in swimming pools where maintaining the proper pH levels is essential for water quality. Ideal pH in swimming pools typically ranges from 7.2 to 7.8. Correcting pH levels can be achieved through pH adjustments using various chemicals accordingly. Failing to maintain appropriate pH can result in harmful bacteria proliferation and adverse effects on the pool’s surface and equipment.
Conclusion
Knowing how to calculate pH accurately is integral across multiple fields such as chemistry, biology, environmental science, and even everyday applications. With several available pH testing methods, anyone can ensure that they are measuring accurately, whether it be in an academic setting, at home, or in industry. The implications of wrong pH levels can lead to various issues, so managing and adjusting pH effectively remains paramount. By implementing this simple guide, readers can gain a deep understanding of how to calculate pH and its relevance.
FAQ
1. What is the importance of knowing how to calculate pH?
Understanding how to calculate pH is crucial since pH levels affect chemical reactions, biological processes, and environmental health. It helps in agricultural practices, water quality testing, and also ensures proper functioning in pharmaceuticals. Whether managing pH levels in pools, aquariums, or food production, accurate pH measurement techniques are vital for optimal outcomes.
2. How can I adjust the pH of my soil?
To adjust soil pH, you can either raise or lower it depending on your soil’s current acidity. To lower pH, typically add sulfur or peat moss; to raise it, use lime. Regular testing with a suitable pH testing kit will inform you of how much adjustment is needed for optimal plant health.
3. What is a buffer solution and its significance?
A buffer solution is a liquid that resists changes in pH when small amounts of acid or base are added. Buffer solutions are crucial in biological and chemical applications as they maintain a stable environment, which is particularly important for reactions involving pH and enzymes during fermentation or metabolic processes.
4. How do pH levels influence fermentation?
The pH in fermentation must be controlled as it affects yeast and bacterial activity, thereby influencing the flavor and quality of the final product. Yeast thrives in slightly acidic environments, typically around pH 4 to 4.5, crucial for making bread, beer, and vinegar.
5. What effect does temperature have on pH readings?
Temperature can affect pH readings since the solubility of gases and the kinetic energy of molecules increases, causing fluctuations in ion activity. Therefore, it’s important to use temperature compensation if available in your pH meter to ensure accuracy in readings.
“`